from UConn Today

Yu Lei, professor of chemical and biomolecular engineering, has invented a new platform that can perform high-sensitivity readings for a variety of disease biomarkers.
In the 1970s, scientists invented the enzyme-linked immunosorbent assay (ELISA). Since then, ELISA has been the standard for detecting biomarkers.
Biomarkers are molecules present in the body that indicate the presence or severity of a disease. For example, autoantibodies can help detect autoimmune diseases, or a peptide known as amyloid-β can indicate Alzheimer’s disease.
One of the major limitations for ELISA is that if there is a low concentration of the molecule of interest, it cannot detect it. Lei’s invention addresses this problem by adding two amplification steps to ELISA’s process.
“We wanted to bridge the need for ultra-sensitivity, and also compatibility with the existing plate-based platform,” Lei says.
ELISA works using a “sandwich” of two antibodies specifically designed to capture/detect the biomarker of interest between them. One of these antibodies has an enzyme attached to it that will produce a readable signal when it encounters the substrate.
Lei introduced a two-step amplification to the ELISA reaction. Lei first added a tyramide signal amplification (TSA) process to amplify the signal of a low abundance biomarker. In Lei’s platform, the TSA step anchors numerous biotins onto the immunocomplexes. Lei then introduced the reporter enzyme alkaline phosphate (ALP) conjugated with streptavidin, which attaches to the biotins through the strong interaction between biotin and streptavidin.
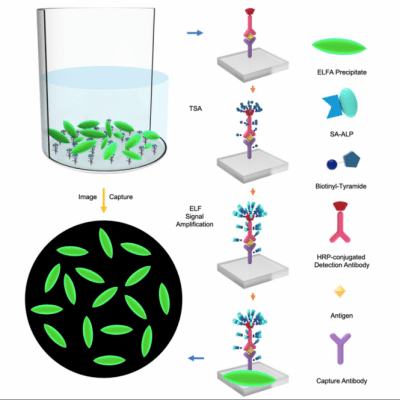
Lei added an ELFA-saturated ELFP substrate that ALP breaks down to produce a fluorescent signal. These molecules that precipitate through the system to form a readable layer consisting of fluorescent needles that a microscope captures as a series of images and counts. This fluorescent microneedle count corresponds to how much of the biomarker is in the sample.
“That’s the beauty of the system using ELFA-saturated ELFP substrate and counting-based method, we achieved rapid detection and at the same time no matter your initial number of target molecules their precipitating time is starting from the same point,” Lei says.
Lei successfully demonstrated that his process was able to achieve a resolution of 50 to 60 picograms per milliliter. This is about 20 times more sensitive than traditional ELISA using the same commercial ELISA kit.
Lei published his findings in the March issue of Analytica Chimica Acta.
This advancement could be extremely useful for early-stage detection of diseases and treatment.
“A lot of disease detection occurs when symptoms are already onset,” Lei says. “That biomarker concentration is already very high. So then, if we can detect at a very low concentration, we can capture the earliest stage and treatment may be more effective.”
Lei says the next step for this technology is to smoothly integrate it into conventional plate-based ELISA systems. This would allow the process, which currently takes about four hours for low-abundance biomarker detection, to be much faster by using advanced imaging systems.